Professional Medical Equipment Manufacturing
And Technical Solution Provider
Thank you for visiting Daeyang Medical’s official website. Daeyang Medical is a medical equipment manufacturing and technical solution provider that develops innovative products that lead the market in the field of aesthetic / physical / rehab. treatment by appropriately combining the medical device manufacturing know-how of the past 50 years with the latest modern technology.
Sparking the Evolution
Our innovative approach and entrepreneurship are over 50 years old.
1970

Sep.
Establishment of Daeyang Medical
1995

Sep.
Permitted as a medical equipment
manufacturer by KFDA
2000

Sep.
Head office and factory
relocation to Bucheon city
2002

Sep.
Incorporation of
Daeyang Medical Co., Ltd
2005

Apr.
ISO9001 / ISO13485 certified
medical device development
and manufacturing certification
2006

Nov.
Head office and factory
relocation to Wonju city
Aug.
Medical device manufacturing
approval by KFDA
2007

Jun.
Promising export small business certification
Venture business certification
Designated by the Korean government
2008

Dec.
World Class Product Award
By the Korean government
2009

Apr.
Certified a Mark of Quality,
High Technology and Trustworthiness
by the Korean government
Jul.
Certified a small and medium-sized enterprise
with high growth potential by the Government of
Gangwon province
2010

Sep.
Celebrated
40th Anniversary of foundation
2011

Jun.
Received Great Place
to Work Award
Jun.
Obtained CAMCAS for
the designand manufacture
of medical devices by SGS
2012

Dec.
Received a citation for
an outstanding cooperative company
2013

Mar.
Appointed as Gangwon
IP Star Corporation
2016

Jan.
5 million USD Export Achieved
2018

Jan.
FDA Approved RF technology
Contactless Selective RF device Launched
2020

Sep.
Celebrated 50th Anniversary of foundation
Nov.
10 million USD Export Achieved
Business Areas

Aesthetic Division
We develop and manufacture professional medical equipment related to medical aesthetic, body contouring and fat reduction. We will continue to research the latest global beauty technology trends and grow as a global leader in the medical aesthetics market.
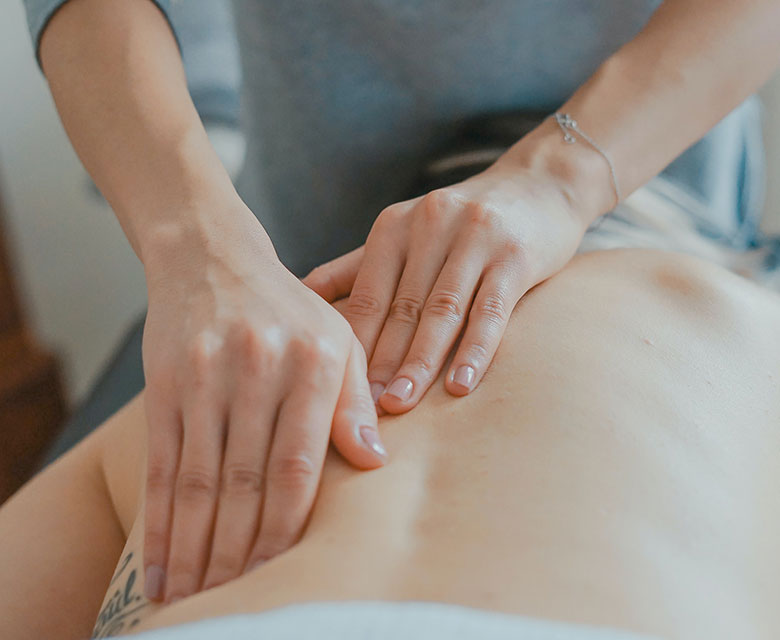
Physiotherapy Division
We have secured source technology for high frequency thermal therapy that has been clinically recognized for a long time, and we are developing various treatment protocols and applying them to our products. We will continue to strive to develop safe professional medical devices.

OEM & ODM
Through the worldwide network of Daeyang Medical Co., Ltd., which has been accumulated over the past 50 years, we are conducting joint development, manufacturing, production and supply of products with various global companies. If you are interested, please leave an inquiry on the website and we will contact you as soon as possible.

Development Division
In order to develop and supply safe medical devices that meet global standards, the development of appropriate medical electrical and electronic modules is an essential part. Daeyang Medical Co., Ltd. has a lot of experience and know-how as it has steadily supplied related technologies to the market for decades.
Certifications & Awards
CE(0068)

CE(1984)

Ministry of
Food and Drug Safety

LL-C(Certification)
_ISO 13485(2016)

US Food and Drug
Administration

KGMP

A Mark of Quilty,
High-Technology&
Trustworthiness

World Class Product
of Korea Award
